发布于:2015-10-08
南极鱼类在南极海洋生态中占据重要的地位。几千万年来在冰冻和极端寒冷的选择压力下,南极鱼类发生了多种多样的基因水平和生理水平的变化。揭示这些变化的遗传基础是研究生物如何适应环境的重要内容。在众多的南极鱼类中,冰鱼科鱼类是目前已知的唯一缺乏血红蛋白和功能性血红细胞的脊椎动物。Nature 杂志曾专栏阐述南极冰鱼可作为贫血症、血细胞生成失调等疾病的研究模型。而迄今为止,南极冰鱼的血红细胞如何丢失以及是否具有适应意义是一个还未阐明的科学问题。
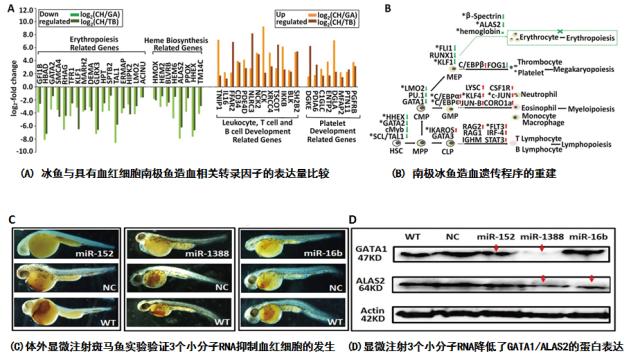
我校科研人员利用跨物种的转录组与小分子RNA组比较,揭示出南极冰鱼在造血程序上发生了大规模的基因调控的重建。它们在负责造血的组织(如头肾)中,一方面强力抑制了与造血相关的因子如GATA1,ALAS2等的表达,同时调高了上百个microRNA的表达。实验表明这些上调的microRNA很大部分与抑制造血转录因子有关。更有意思的是,课题组研究人员首次发现:造血转录因子的强力抑制以及microRNA表达的普遍调高都是由一个相同的信号通路,即TGF-β信号通路的上调所引起的。进化分析表明,TGF-β信号通路中的两个重要因子,TGF-β1和endoglin在南极鱼亚目鱼类的进化中发生了正选择,而这种正选择作用发生在南极鱼亚目鱼类进入南极后形成的所有科属的鱼类中,而不仅仅局限于血红蛋白丢失的冰鱼科鱼类中。这表明,南极鱼类在进入低温的南极海域后就发生了调高TGF-β信号通路的进化趋势。这种趋势导致生活在南极的鱼类有血红细胞减少的普遍趋势,而这种趋势在冰鱼科鱼类中表现更为强烈。因此,冰鱼血红细胞的丢失具有对低温环境适应的进化意义。本项工作在分子水平深化了人们对鱼类血液细胞发生与低温适应之间关系的认识。
该项研究成果已于2015年9月6日在线发表于国际分子生态学领域的顶级期刊Molecular Ecology上,影响因子为6.494。海洋科学学院的许强华教授为该论文的第一作者,水产与生命学院陈良标教授与许强华教授为共同通讯作者。课题组多位研究生、博士后和多位工作人员参加了研究。该研究得到国家自然科学基金重大研究计划培育项目,上海市教育发展基金会与上海市教育委员会“曙光计划和上海高校水产学一流学科建设项目等资助。
论文下载:http://onlinelibrary.wiley.com/doi/10.1111/mec.13344/abstract
(撰稿:翟万营)
Abstract:
Evolutionary suppression of erythropoiesis via the modulation of TGF-β signalling in an Antarctic icefish
Xu Q1,2, Cai C1, Hu X1, Liu Y1, Guo Y1, Hu P3, Chen Z3, Peng S3, Zhang D3, Jiang S3, Wu Z3, Chan J1, Chen L3
The Antarctic icefish, a family (Channichthyidae) of teleosts within the perciform suborder Notothenioidei, are the only known vertebrates without oxygen-transporting haemoglobins and that are largely devoid of circulating erythrocytes. To elucidate the evo-devo mechanisms underpinning the suppressed erythropoiesis in the icefish, we conducted comparative studies on the transcriptomes and microRNAomes of the primary haematopoietic tissues between an icefish (Chionodraco hamatus) and two red-blooded notothenioids (Trematomus bernacchii and Gymnodraco acuticeps). We identified substantial remodelling of the haematopoietic programs in the icefish through which erythropoiesis is selectively suppressed. Experimental verification showed that erythropoietic suppression in the icefish may be attributable to the upregulation of TGF-β signalling, which coincides with reductions in multiple transcription factors essential for erythropoiesis and the upregulation of hundreds of microRNAs, the majority (> 80%) of which potentially target erythropoiesis regulating factors. Of the six microRNAs selected for verification, three miRNAs (miR-152, miR-1388 and miR-16b) demonstrated suppressive functions on GATA1 and ALAS2, which are two factors important for erythroid differentiation, resulting in reduced numbers of erythroids in microinjected zebra fish embryos. Codon substitution analyses of the genes of the TGF-β superfamily revealed signs of positive selection in TGF-β1 and endoglin in the lineages leading to Antarctic notothenioids. Both genes are previously known to function in erythropoietic suppression. These findings implied a general trend of erythropoietic suppression in the cold-adapted notothenioid lineages through evolutionary modulation of the multi-functional TGF-β signalling pathway. This trend is more pronounced in the haemoglobin-less icefish, which may pre-emptively hinder the otherwise defective erythroids from production.